Around 9,100 people are diagnosed with oesophageal cancer each year in the UK, and 22 patients with this diagnosis die every day.
One of the most challenging things about treating this cancer is that it is usually diagnosed at an advanced stage, and the early symptoms are often mistaken for heartburn. This means that prognosis is poorer, with fewer treatment options available to patients.
Some people develop a condition called Barrett’s oesophagus, when cells in the gullet grow abnormally, prior to developing oesophageal cancer. Barrett’s is much more common than oesophageal cancer. Although it will only develop into cancer in a handful of cases, identifying it early presents an opportunity for doctors to monitor the condition and intervene before cancer develops.
About the project
For the past several years, researchers at the Medical Research Council (MRC) Cancer Unit in Cambridge led by Professor Rebecca Fitzgerald have been developing a simple and inexpensive test called the Cytosponge-TFF3 test. The test can detect abnormal cells in the gullet, which may indicate a person is developing Barrett’s, or oesophageal cancer.
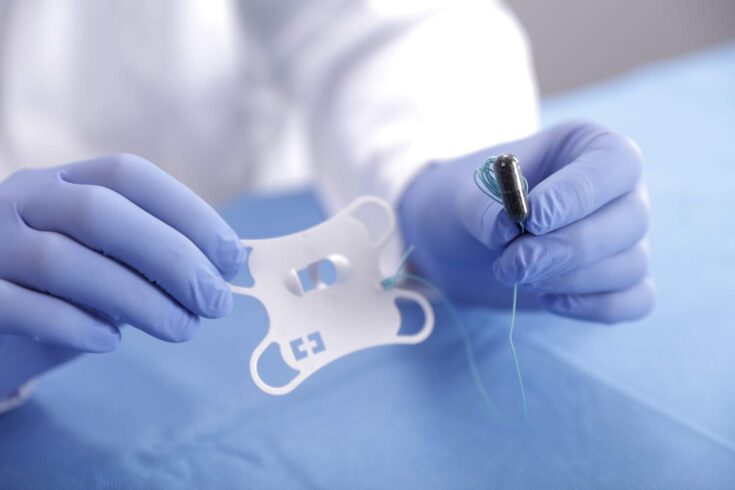
The Cytosponge is a small round sponge contained within a dissolvable capsule. It is attached to a piece of string, and is swallowed with water. The capsule dissolves in the stomach to expand into a sponge-like mesh which is 3cm wide. It is then pulled back up using the string, collecting cells on the way, which are analysed in the lab using a test specially designed by Fitzgerald and her team
Unlike an endoscopy, the Cytosponge does not require an invasive test, which is uncomfortable for the patient. It also only costs £25 and takes just 10 minutes to administer in a GP clinic without the need for a hospital visit.
The team completed early clinical assessment of the technique in 2010, where the findings showed that the Cytosponge test provided an accurate and less uncomfortable method of diagnosis.
Cancer Research UK then funded a large-scale study to follow up these findings and found the test to be well tolerated, safe, and accurate. They showed that when the two methods (endoscopy and Cytosponge) were compared, the Cytosponge had similar accuracy in diagnosing Barrett’s as an endoscopy.
Impacts of the project
In October 2020, the Cytosponge test was introduced in Scotland as a ‘simpler alternative to endoscopy’ in a £500,000 programme. NHS Greater Glasgow and Clyde and NHS Lanarkshire were the first to implement the test.
Less than a year later in July 2021, Scotland announced that the Cytosponge test will now be used across all mainland Scottish health boards in the surveillance of people with Barrett’s oesophagus.
Find out more
Read about the use of Cytosponge as a triaging tool to upper gastrointestinal endoscopy during the COVID-19 pandemic in The Lancet.
Top image: The Cytosponge is contained in a dissolvable capsule that is swallowed. Credit: Medtronic.